

Ca (OH)2 is slightly soluble in water with approximately 1 g Ca (OH)2 dissolving in a liter of water. Oxygen Preparation Carbon Dioxide Nitrogen and Ammonia Sulfur, its oxides and Sulfuric Acid Group 7. Alkaline Earth Metals Aluminium Zinc Iron Lead Copper Tests for Cations Q & A. After several minutes, some bubbles of hydrogen form on its surface, and the coil of magnesium ribbon usually floats to the surface. Mg (s) + H 2 O (g) MgO (s) + H 2 (g) Very clean magnesium ribbon has a very slight reaction with cold water.
Magnesium burns in steam to produce white magnesium oxide and hydrogen gas. $\begingroup$ You need to use the Nernst equation to adjust the potentials accordingly to the much higher pH value. I see no reason for it to not react with hot sodium hydroxide (though it might happen slower). $\begingroup$ Magnesium does react with hot water (not so much with cold water). Calculate the heat lost by the warm water and the heat gained by the cold water (mass H2O * ∆T * specific heat.). water quickly and as completely as possible into the other calorimeter, and take a reading of the combined water after another minute has elapsed using the thermometer from the cooler water calorimeter. By In collector's edition games 2022 Mano comments. (i) Write an equation for the reaction of magnesium with cold water. With sodium the solution is clear but with magnesium it appears cloudy. (a) Sodium and magnesium both react with cold water to produce the same type of product in solution. H-F is a weak acid (~2.5% ionized) in aqueous media where as HCl, HBr & HI are strong acids ionizing 100% in aqueous media b/c of decreasing H/X bond strengths.1 Sodium and magnesium are the first two elements in the third period. The numerical values (supported by many online references) are. As a result the bond energy trends, from strongest to weakest follow H-F > H-Cl > H-Br > H-I. As atomic number increases additional energy levels are present causing a shielding effect that reduces the influence of the nucleus on the valence level electrons. Such is why the trend is called 'The Hydrogen Halide Paradox'.
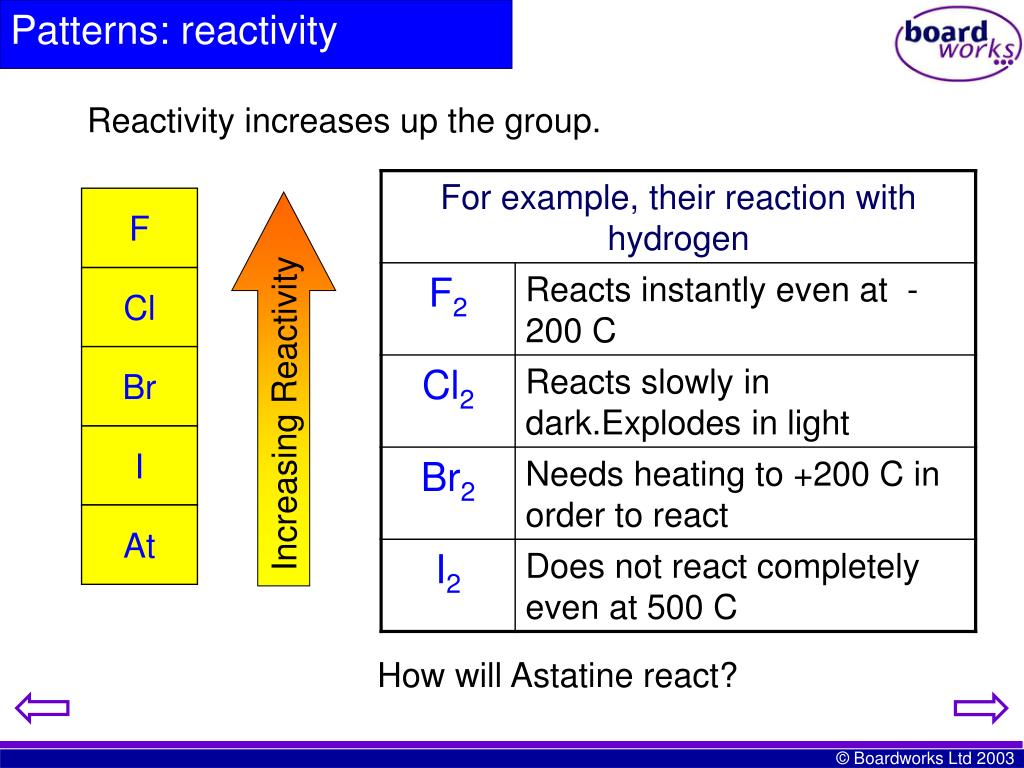
#Halogens reactivity series#
The bonding energies of the H-X series are 'size of halide ion' dependent, not 'electronegativity' dependent as in series substances.

'Down' the group the strength of H-X bonds decrease and form weaker bonds giving up 'less' energy on formation. As a result due to difference in energy requirements the reactivity of H-X is higher up the group The acidity of the Hydrogen - Halide group is also referred to as the 'Hydrogen Halide Paradox'Ĭan you please also tell me what role does the bond enthalpy of H-X plays in the reactivity trends of halogens towards hydrogen?ĭown the group the strength of H-X bonds increases and to form a strong bond more energy is required. The order of increasing electronegativity is C (H - Cl) > (H - Br) > (H - I) due to the addition of principle energy levels (rings) creating a 'shielding effect', 'Shielding' decreases the electrostatic influence of the nucleus on the bond between the valence of the anion and the attached substrate. Ionizations follow the same trends in series for the Group I - III elements, but C, N, O & F tend to gain electrons causing a large in crease in ionic radius after Boron because of e-/e-repulsion, but electronegative effects still increase from Carbon through Fluoride. The effect of increasing electronegativity of neutral elements in series functions to decrease atomic radius b/c of increased force of electrostatic attraction. Electronegativity increases with increasing atomic number due to increasing number of electrons and protons making up the atomic and ionic structures.

In series (consider Series II => Li, Be, B, C, N, O, F & Ne). Bond Energy trends are a function of electronegativity in series and of ionic size in groups.


 0 kommentar(er)
0 kommentar(er)
